- Schedule 4 Narcotics Definition
- Class 3 Narcotic List
- List Of Schedule 5 Drugs
- Schedule 3 Narcotic List
Schedule 4 Narcotics Definition
The following Schedule III, IV, and V non-narcotic controlled substances have been specifically designated by the Administrator of the Drug Enforcement Administration as requiring import and export permits pursuant to sections 201(d)(1), 1002(b)(2), and 1003(e)(3) of the Act (21 U.S.C. 811(d)(1), 952(b)(2), and 953(e)(3)):
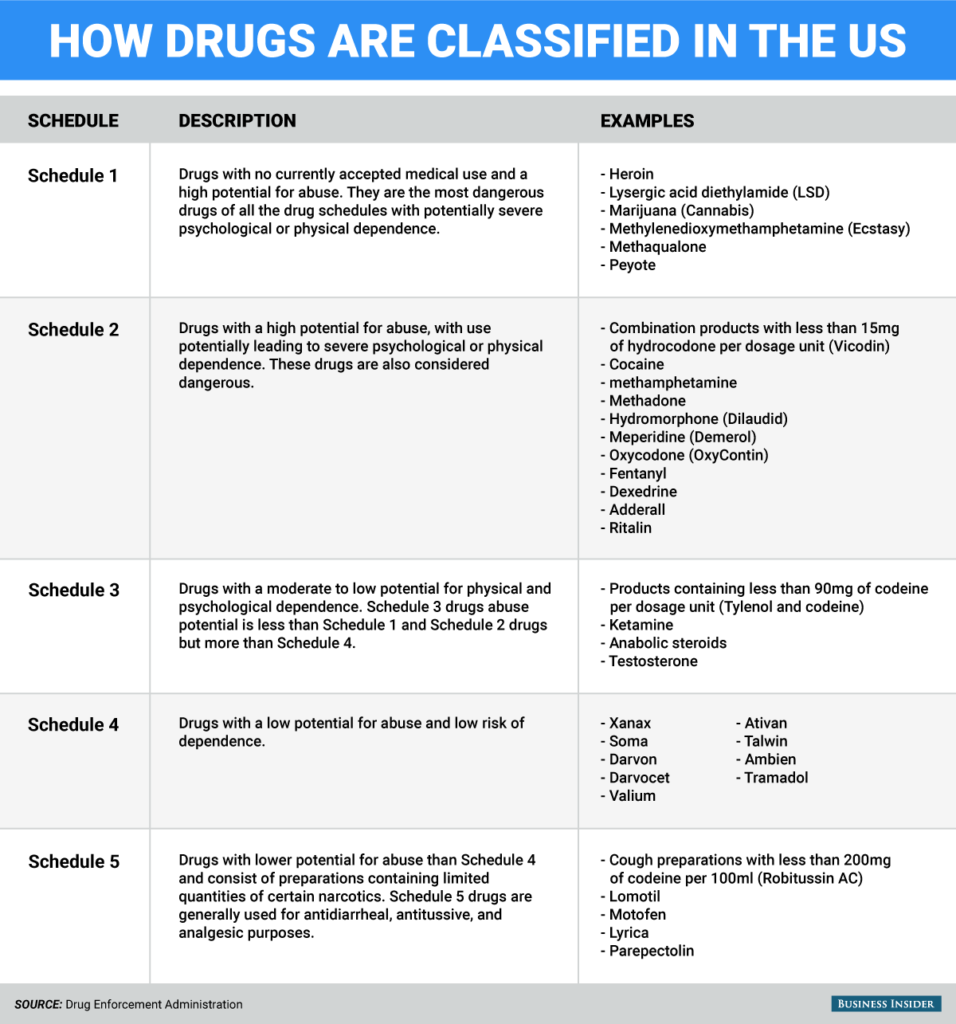
Schedule III drugs abuse potential is less than Schedule I and Schedule II drugs but more than Schedule IV. Some examples of Schedule III drugs are: Products containing less than 90 milligrams of codeine per dosage unit (Tylenol with codeine), ketamine, anabolic steroids, testosterone.
The drug or other substance has a high potential for abuse, and has no currently accepted medical. Some drugs have been reclassified over the years. For example, in 2014, the DEA reclassified the drug hydrocodone, moving it from Schedule III to Schedule II. But on the whole, reclassification or unscheduling a substance is rather rare, and this has led to many controversies surrounding the Controlled Substances Act. Amendment adding drug products in finished dosage formulation that has been approved by the U.S. Food and Drug Administration that contains cannabidiol (2-1R-3-methyl-6R-(1-methylethenyl)-2-cyclohexen-1-yl-5-pentyl-1,3-benzenediol) derived from cannabis and no more than 0.1 percent (w/w) residual tetrahydrocannabinols to Schedule V Effective.
Class 3 Narcotic List
(a) Dronabinol (synthetic) in sesame oil and encapsulated in a soft gelatin capsule in a U.S. Food and Drug Administration approved product.

List Of Schedule 5 Drugs
(b) [Reserved]